Answer:
More neutrons than protons.
Step-by-step explanation:
Hello,
In this case, it is necessary to know that in an atom, the number of protons are equal to the number of electrons based on the classic atomic theory. On the other hand, protons are equal to the atom's atomic number and neutrons are computed as the difference between the atom's atomic mass and the atom's atomic number, for instance, for ruthenium:
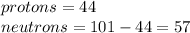
In such a way, an atom tends to have more neutrons than protons but the difference is even greater for elements with high atomic numbers.
Best regards.