Answer: 12.4
Explanation: pH or pOH is the measure of acidity or alkalinity of a solution.
Acids have pH ranging from 1 to 6.9 and bases have pH ranging from 7.1 to 14.Neutral substances have pH of 7.
First we have to calculate the pH.
![pH=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/y1nlg9qxar6fauop1r05a1g4xt6dhnvirc.png)
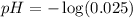
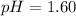
Now we have to calculate the pOH.
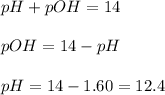
Thus pOH of the solution is 12.4