the final temperature of the system is 32.5°
we know, H = mcT
where, H = Heat content of the body
m = Mass,
c = Specific heat
T = Change in temperature
According to to the Principle of Calorimetry
The net heat remains constant i.e.
⇒ the heat given by water = heat accepted by the aluminum container.
⇒ 330 x 1 x (45 - T) = 855 x
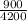
x (T - 10)
⇒ 14,850 - 330T = 183.21T - 1832
⇒ - 513.21 T = - 16682
or T = 32.5°