1) since we have percentages, we can assume we have 100 grams of the compound.
85.6% Carbon---> 85.6 grams of C
14.4% Hydrogen--> 14.4 grams of H
2) we need to convert the grams to moles for each one using their molar masses.
molar mass of C= 12.0 g/mol
molar mass of H= 1.01 g/mol
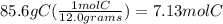
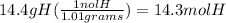
3) divide each mole by the smallest value(7.13) to find the ratios
Carbon--->

Hydrogen--->
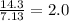
4) use these ratios to write the empirical formula.
CH₂