Step-by-step explanation:
It is known that water has molecular formula
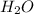 . Since oxygen atom is electronegative in nature and hydrogen atom is electron deficient in nature.
. Since oxygen atom is electronegative in nature and hydrogen atom is electron deficient in nature.
Therefore, oxygen atom will attract the electron of hydrogen atom and thus it develops a partial negative charge and hydrogen atom will develop a partial positive charge.
Thus, we can conclude that a partial negative electrical charge is found on the oxygen atom of a polar water molecule.