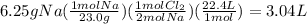balanced equation: 2Na(s) + Cl₂(g) ---> 2NaCl(s)
when we have STP conditions, we can use this conversion: 1 mol = 22.4 L
first, we have to convert grams to molecules using the molar mass, and then use mole to mole ratio from the balanced equation.
molar mass of Na= 23.0 g/mol
ratio: 2 mol Na= 1 mol Cl₂ (based on coefficients of balanced equation)
calculations: