Answer: 12.4 %
Step-by-step explanation:
To calculate the mass percent of solution, we use the formula:
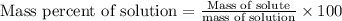
Given :
mass of solute (LiCl) = 31.8 grams
mass of solvent (water) = 225 grams
Mass of solution = mass of solute + mass of solvent = 31.8 g + 225 g= 256.8 g
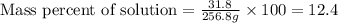
Thus concentration of the solution in percent by mass is 12.4%