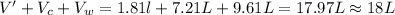Answer:
The correct answer is option D.
Step-by-step explanation:
At room conditions ,temperature is taken as T =293.15 k
At room conditions, pressure is taken as P = 1 atm
Given the volume of oxygen gas =
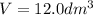 = 12.0 L
= 12.0 L
Using Ideal gas equation:
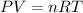
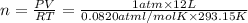
n = 0.4992033 moles of oxygen gas.
Initial number of moles of oxygen gas = 0.4992 mol
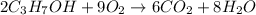
According to reaction, 2 moles of propanol reacts with 9 moles of oxygen.
Then 0.10 moles of propanol will react with:
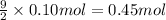
Final moles of oxygen gas left after combustion reaction = n'
n'= 0.4992 mol - 0.45 mol = 0.0492 mol
So, the final volume of the oxygen gas measured will be given as:V'
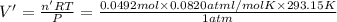
V' = 1.18 L
Volume of the
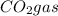
According to reaction, 2 moles of propanol gives with 6 moles of
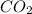 .
.
Then 0.10 moles of propanol will give :
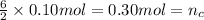
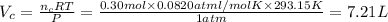
Volume of the
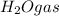
According to reaction, 2 moles of propanol gives with 8 moles of
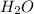 .
.
Then 0.10 moles of propanol will give :
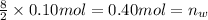
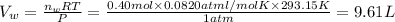
Total volume of the gas measured =