Answer:
The change in temperature is 26.4 C
Step-by-step explanation:
Given:
Mass of the substance, m = 10.3 g
Specific heat of the substance, c = 2.01 J/g C
Heat absorbed, q = 542 J
To determine:
The change in temperature, ΔT
Step-by-step explanation:
The amount of heat (q) absorbed (or evolved) by a substance of mass (m) in order to bring a temperature change of (ΔT) is given as:
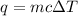
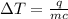
In this case:
