Answer: 3. a salt
Step-by-step explanation:
A double displacement reaction is one in which exchange of ions take place. The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.
Neutralization reaction is defined as a double displacement reaction in which an acid reacts with a base to produce salt and water.

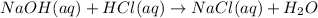
When the aqueous solution of salt and water is evaporated, the liquid water will convert to gaseous state and no volatile salt will remain as such.