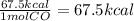when the enthalpy value is given, we can calculate how much heat is use or produces in a given equation.
67.6 kCal ---> 67.6 kCal= 1 mol of reaction
1 mol of reaction= 1 mol of CO (based on the coefficient)
so 1 mole of CO gives us 67.6 kCal of heat.
calculation:
1 mol CO