1) since we are given percentages, we can assume we have 100 grams of the molecule.
55.6 % Cu ----> 55.6 grams Cu
16.4 % Fe------> 16.4 grams Fe
28.0% S--------> 28.0 grams S
2) convert each gram to moles using the molar masses given
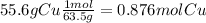
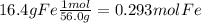
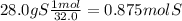
3) we divide the smallest value of moles (0.293) to each one.
Cu --> 0.876 / 0.293= 3
Fe---> 0.293 / 0.293= 1
S-----> 0.875 / 0.293= 3
4) let's write the empirical formula
Cu₃FeS₃