Answer: The addition of
 will cause a shift in the given equilibrium equation.
will cause a shift in the given equilibrium equation.
Step-by-step explanation: We are given a chemical equation:
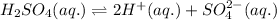
Common ion effect is the effect seen when there is an addition of one common ion to the equilibrium conditions and it effects the equilibrium to counteract the effect of addition.
In the given equilibrium,
 will cause a shift in the equilibrium because of the presence of
will cause a shift in the equilibrium because of the presence of
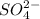 ions in both the reactions.
ions in both the reactions.
Ionization of
 is given by:
is given by:

As the
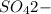 ions are added to the right side, so to counteract the effect of this, the equilibrium will shift in the left direction that is the reaction will proceed in the backward direction.
ions are added to the right side, so to counteract the effect of this, the equilibrium will shift in the left direction that is the reaction will proceed in the backward direction.