Answer: The required number of grams needed is 15 grams.
Step-by-step explanation: Given that a solution has a density of 1.50 g/mL.
We are to find the number of grams that are needed to obtain 10.0 mL of solution.
We will be using the UNITARY method to solve the problem.
We have
Number of grams needed o obtain 1 mL of solution = 1.50 gm.
So, number of grams needed to obtain 10.0 mL of solution is given by
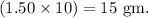
Thus, the required number of grams needed is 15 grams.