Answer : The correct option is, (C) 153
Explanation :
Atomic number is defined as the number of protons or number of electrons.
Atomic number = number of protons = number of electrons
Mass number is defined as the sum of number of protons and number of neutrons.
Number of neutrons = Mass number - Number of protons
or,
Number of neutrons = Mass number - Atomic number
As we are given that:
The given nuclide is,
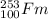
'Fm' is the fermium element.
Mass number of 'Fm' = 253
Atomic number f 'Fm' = 100
Now we have to determine the number of neutrons.
Number of neutrons = Mass number - Atomic number
Number of neutrons = 253 - 100
Number of neutrons = 153
Therefore, the number of neutrons in the 'Fm' nuclide have 153.