Answer:
Coefficient of water = 2
Step-by-step explanation:
A chemical reaction is said to be balanced when there are equal number of atoms/elements of one type on both the reactants and products side.
The given reaction is:
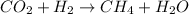
# Atoms in Reactants # Atoms in Products
C = 1 C = 1
O = 2 O = 1
H = 2 H = 6
The equation is not balanced since the number of O and H atoms are 2 each on the reactants and 1 and 6 respectively on the products side. In order to balance it, multiply H2 by 4 and H2O by 2 to get:
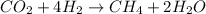
Therefore, the coefficient of H2O = 2