Answer : Trigonal Planar.
Explanation : The molecular geometry of
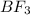 is trigonal planar with symmetric charge distribution on the central atom of Boron. It has three bonds with Boron as the central atom and Fluorine as the peripheral atoms. The molecule which has central atom as boron does not contains any lone pairs on it.
is trigonal planar with symmetric charge distribution on the central atom of Boron. It has three bonds with Boron as the central atom and Fluorine as the peripheral atoms. The molecule which has central atom as boron does not contains any lone pairs on it.
Therefore, it has trigonal planar geometry. As the fluorine atoms are arranged in a form of triangle placing boron in the center.