Answer:
We will determine the weighted average of the atomic mass of two isotopes.
Step-by-step explanation:
The atomic mass of an element is the weighted average of atomic masses of the most abundant isotopes occur in nature.
Given:
Percentage abundance of C-12 : 98.89%
Percentage abundance of C-13: 1.108%
The atomic mass of carbon will be"
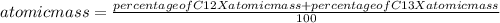
Atomic mass =
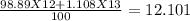 g/mol
g/mol