The concentration of
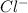
ions will be double than that of
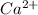
Calcium chloride -
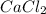
has one Ca atom and 2 atom of chlorine.
When it is dissolved in water, it disassociates into
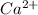
and
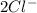
Thus, the concentration of
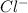
ions will be double than that of
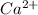
Or we can say the ratio of
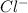
:
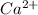
= 2:1