Step-by-step explanation:
Specific heat of the substance indicates the amount of heat or energy required to raise the temperature of the unit mass of the substance by one unit degree Kelvins.
It is measured in joules per gram degree Kelvin or
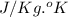 .
.
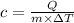
Q = Amount of heat absorbed by the substance in joules
c = Specific heat of the substance
m = Mass of substance in Kg
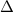 T = Change in temperature in Kelvins
T = Change in temperature in Kelvins