Answer: The correct answer is nitric acid.
Step-by-step explanation:
An acid is defined as the substance which looses hydrogen ions when dissolved in water.
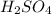 is an acid because it looses 2 hydrogen ions when dissolved in water.
is an acid because it looses 2 hydrogen ions when dissolved in water.
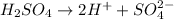
A base is defined as the substance which looses hydroxide ions when dissolved in water or it accepts hydrogen ions easily.
An acid is neutralized only by base.
From the given options:
Option 1: Sodium hydroxide
The chemical formula for this compound is NaOH. This compound will donate hydroxide ion when dissolved in water. Therefore, this is a base.
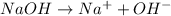
Option 2: Nitric acid
The chemical formula for this compound is
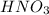 . This compound will donate hydrogen ion when dissolved in water. Therefore, this is an acid.
. This compound will donate hydrogen ion when dissolved in water. Therefore, this is an acid.

Option 3: Lithium hydroxide
The chemical formula for this compound is LiOH. This compound will donate hydroxide ion when dissolved in water. Therefore, this is a base.
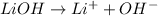
Option 4: Ammonia
The chemical formula for this compound is
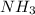 . This compound will accept hydrogen ion. Therefore, this is a base.
. This compound will accept hydrogen ion. Therefore, this is a base.
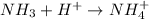
Hence, the correct answer is nitric acid.