Answer: spontaneous at high and low temperatures
Explanation:
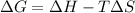
 = Gibb's free energy change
= Gibb's free energy change
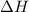 = enthalpy change
= enthalpy change
T = temperature
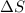 = entropy change
= entropy change
A reaction is spontaneous when
 = Gibb's free energy change is negative.
= Gibb's free energy change is negative.
Given
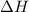 = decrease in enthalpy = -ve
= decrease in enthalpy = -ve
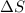 = increase in entropy = +ve
= increase in entropy = +ve
Thus
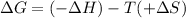

As
 is always negative, it is spontaneous at all the temperatures.
is always negative, it is spontaneous at all the temperatures.