Answer: Option (a) is the correct answer.
Step-by-step explanation:
A bond that is formed because of transfer of electrons from one atom to another is known as an ionic bond.
For example, sodium has 1 valence electron and chlorine has 7 valence electrons. So, transfer of one electron from sodium to chlorine will result in an ionic bond leading to the formation of compound NaCl.
Also, there is large difference in electronegativities of a metal and a non-metal. Usually, a metal and a non-metal form an ionic bond.
On the other hand, a bond formed by sharing of electrons is known as a covalent bond.
For example,
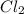 molecule has covalent bond.
molecule has covalent bond.
Hence, we can conclude that bonding atoms that experience large differences in electronegativity values will experience ionic bond.