Answer:
If pressure decreases density decreases and increase the pressure if the pressure increases.
Step-by-step explanation:
From the ideal gas law we have
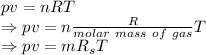
Now divide both sides by volume we get
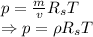
Where,
R = Gas constant
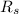 = Mass-specific gas constant
= Mass-specific gas constant
T = Temperature of gas
n = Number of moles of gas
m = Mass of gas
v = Volume of gas
p = Pressure of gas
ρ = Density of gas
It can be seen from the equation that the pressure and density are proportionally related.
If pressure decreases density decreases and increase the preusure if the pressure increases.