Answer : The density of a gas is, 1.013 g/L
Solution : Give,
Molar mass of gas = 16.01 g/mole
Pressure of gas = 1.75 atm
Temperature of gas = 337 K
Using ideal gas law equation,
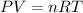
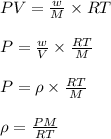
As we know that
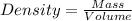
where,
P = pressure of the gas
V = volume of the gas
T = temperature of the gas
n = number of moles of the gas
w = given mass of gas
M = molar mass of gas
R = gas constant = 0.0821 Latm/moleK
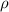 = density of gas
= density of gas
Now put all the given values in the above formula, we get the density of the gas.
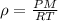
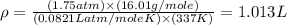
Therefore, the density of a gas is, 1.013 g/L