Answer : The number of moles of hydrogen in 2.7 mole of caffeine are 27 moles.
Explanation : Given,
Moles of caffeine = 2.7 mole
The molecular formula of caffeine is,
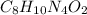
In the molecule
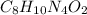 , there are 8 moles of carbon, 10 moles of hydrogen, 4 moles of nitrogen and 2 moles of oxygen.
, there are 8 moles of carbon, 10 moles of hydrogen, 4 moles of nitrogen and 2 moles of oxygen.
As, 1 mole of
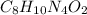 contains 10 moles of hydrogen
contains 10 moles of hydrogen
So, 2.7 moles of
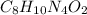 contains
contains
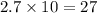 moles of hydrogen
moles of hydrogen
Therefore, the number of moles of hydrogen in 2.7 mole of caffeine are 27 moles.