Answer:
0.24 grams of polonium is in the sample 966 days later.
Explanation:
Given : Suppose a laboratory has a 31 g sample of polonium-210. The half-life of polonium-210 is about 138 days.
To find : How many half-lives of polonium-210 occur in 966 days? How much polonium is in the sample 966 days later?
Solution :
The half-life of polonium-210 is about 138 days.
We have to find the number of half-lives of polonium-210 occur in 966 days.
The number of half-lives is
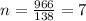
The amount of polonium-210 remaining after t days is given by the equation,
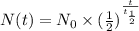
where,
N(t) is the amount of substance remaining after t days,
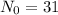 is the initial amount of substance,
is the initial amount of substance,
t=966 is the time in days,
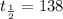 is the half-life of the substance.
is the half-life of the substance.
Substitute the values in the formula,
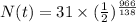
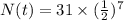
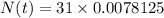
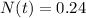
Therefore, 0.24 grams of polonium is in the sample 966 days later.