Answer:

Step-by-step explanation:
To convert from grams to moles, we must use the molar mass found on the Periodic Table of Elements.
1. Molar Mass
This is a sample of sulfur, so look for S on the table.
2. Convert from Grams to Moles
Use the molar mass as a ratio.
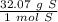
Multiply by the given number of grams: 40
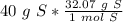
Flip the fraction so the grams of sulfur will cancel each other out.
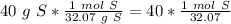
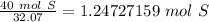
There are 1.24727159 moles of sulfur.