Answer:
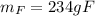
Step-by-step explanation:
Hello!
In this case, given that one molecule of CF2Cl2 contains two moles of atoms of fluorine, we can set up the following dimensional analysis to compute the emitted fluorine each year by the considered car:
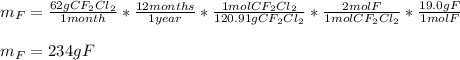
Best regards!