Answer: C) 4
Explanation: Half life is the time in which the amount of a radioactive substance remains halve of its initial amount. For example, half life of carbon-14 is 5730 years. It means if we have 4 grams of carbon-14 then it will remain 2 grams after 5730 years.
For the given problem, the initial amount of a radioactive substance is 64 grams, if 4.0 grams of that substance left, then how many half-lives have elapsed.
So, Its asking to calculate the number of half-lives. The formula used for this is:
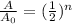
where, A0 is the initial amount and A is final amount and n is the number of half-lives.
Let's plug in the values in the formula.
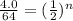
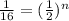
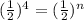
n = 4
So, there will be 4 half-lives and hence C is the correct choice.