Answer:
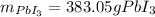
Step-by-step explanation:
Hello!
In this case, since the reaction between lead and iodine is:
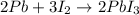
We can see there is 2:2 and 3:2 mole ratio between each reactant and the product; in such a way, for the limiting reactant we must compute the moles of lead (III) iodide yielded by each reactant first:
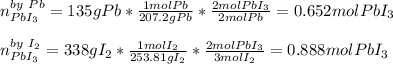
Thus, the limiting reactant is lead because it yields the fewest moles of product. Next, we compute the mass of lead (III) iodide by multiplying the produced 0.652 moles by its molar mass:
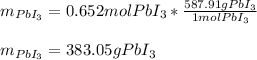
Best regards!