Answer:
PV = nRT
Step-by-step explanation:
The volume expansion of air follows some basic properties of the gas expansion. There are several properties of air that need to be taken into account:
- The volume of air is proportional to the temperature of the air.
The increase in the temperature of the air results in the expansion of the air. This is as a result of the increased kinetic energies of the air particles.
- The number of moles of the gas are the same
- The properties above are expressed by the ideal gas law:
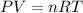
where P = pressure (atm)
n = number of moles (mol)
V = Volume of air (m³)
T = temperature change (K)
R = inversal molar gas constant (atm L/mol K)