Answer: 3 half lives
Solution :
Formula used :
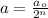
where,
a = amount of reactant left after n-half lives = initial amount - amount of daughter isotope= (1.28-1.12)= 0.16 g
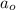 = Initial amount of the reactant = 1.28 g
= Initial amount of the reactant = 1.28 g
n = number of half lives = ?
Putting values in above equation, we get:
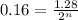
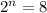
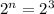
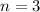
Therefore, 3 half-lives have passed since the sample originally formed.