Answer:
Painting is 13725 years old.
Explanation:
C-14 dating is the process through which age of fossils or dead tissues are determined.
One important thing about the C-14 dating is half life life period of C-14 that is 5730 years.
Now we know for C-14 decay we use the formula
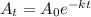
where
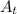 = present quantity of C-14
= present quantity of C-14
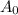 = Initial quantity of C-14
= Initial quantity of C-14
k = Decay constant
t = Duration or time
We will calculate the decay constant first to use this formula for age determination further.
For Half life of C-14
A_{t}=A_{0}e^{-kt}
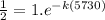
Taking ln on both the sides
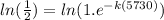
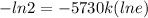
k =
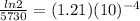
Now we have been given in the question that C-14 found to be 19% and we have to determine the age of the painting.
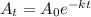
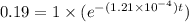
We will take ln on both the sides
![ln(0.19)=ln[1* (e^{-(1.21* 10^(-4))t})]](https://img.qammunity.org/2018/formulas/mathematics/high-school/aik8260tlcb9y5rk17h97q7wux1ur002hq.png)
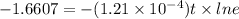
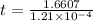
t = 1.3725×
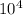
t = 13725 years
Therefore, painting is 13725 years old.