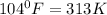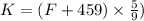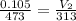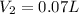Answer: 7. The new temperature of the neon gas is 520 K.
8. The volume of the balloon when it’s placed in a freezer is 2.3 L.
9. The new volume of the gas is 1.25L.
10. The new volume is 0.07 L.
Explanation: Charles' Law: This law states that volume is directly proportional to the temperature of the gas at constant pressure and number of moles.
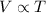 (At constant pressure and number of moles)
(At constant pressure and number of moles)
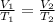
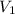 = initial volume of neon gas= 680 ml= 0.68 L
= initial volume of neon gas= 680 ml= 0.68 L
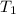 = initial temperature =
= initial temperature =
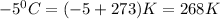
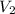 = final volume of neon gas = 1.32 L
= final volume of neon gas = 1.32 L
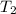 = final temperature = ?
= final temperature = ?
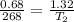
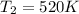
8.
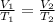
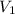 = initial volume of helium gas = 2600 ml = 2.6L
= initial volume of helium gas = 2600 ml = 2.6L
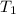 = initial temperature helium gas =
= initial temperature helium gas =

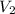 = final volume helium gas=?
= final volume helium gas=?
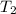 = final temperature of helium gas =
= final temperature of helium gas =
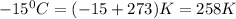
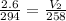
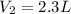
9.
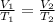
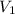 = initial volume of ammonia gas = 650 ml = 0.65L
= initial volume of ammonia gas = 650 ml = 0.65L
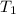 = initial temperature helium gas =T K
= initial temperature helium gas =T K
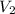 = final volume helium gas=[ ?
= final volume helium gas=[ ?
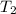 = final temperature of helium gas =
= final temperature of helium gas =
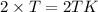
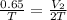
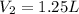
10.
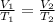
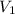 = initial volume =105 ml = 0.105 L
= initial volume =105 ml = 0.105 L
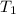 = initial temperature helium gas =
= initial temperature helium gas =
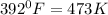
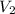 = final volume helium gas= ?
= final volume helium gas= ?
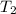 = final temperature of helium gas =
= final temperature of helium gas =