Answer: Student's reasoning is wrong.
Step-by-step explanation:
Carbon is a second period element and its electronic configuration is
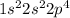 .
.
Since, there is no d-orbital present in a carbon atom. Hence, when it is dissolve in water then it becomes unable to accommodate the lone pair donated by donor oxygen atom of water.
As a result, there occurs no reaction when carbon is dissolved in water.
Hence, we can conclude that we do not agree with the student's reasoning.