Answer:

Step-by-step explanation:
To convert from grams to moles, we must use the molar mass. This can be calculated using the Periodic Table.
1. Molar Mass
First, identify the elements in the compound AlCl₃. They are aluminum and chlorine. Now find the masses:
- Al: 26.981538 g/mol
- Cl: 35.45 g/mol
Take a look at the compound. There is a subscript of 3 after chlorine, so we must multiply its molar mass by 3 then add aluminum's.
- AlCl₃= 3(35.34 g/mol) + 26.981538 g/mol = 133.331538 g/mol
2. Convert Grams to Moles
We can use the molar mass as a ratio.
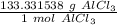
Multiply by the given number of grams: 89.23
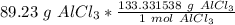
Flip the fraction so the grams of aluminum chloride cancel out.
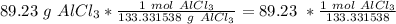
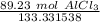
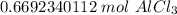
3. Round
The original measurement of grams has 4 significant figures. We must round to 4 sig figs.
For the number we calculated, that is the ten thousandth place.
The 3 in the hundred thousandth place tells us to leave the 2.
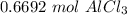
There are about 0.6692 moles of aluminum chloride in 89.23 grams.