Answer : The correct options are,
2. The temperature and the number of molecules must remain constant for the law to apply.
4. As the pressure of a gas increases, the volume decreases proportionally.
Explanation :
Boyle's Law : It is defined as the pressure of the gas is inversely proportional to the volume of the gas at constant temperature and number of moles.
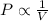
or,
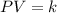
where,
P = pressure of gas
V = volume of gas
k = constant
From this we conclude that, pressure is inversely proportional to the volume of gas that means as the pressure increases, the volume of gas decreases.
Hence, the correct options are, (2) and (4)