Answer:
In 7.05 moles of pyridine there are
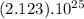 carbon atoms.
carbon atoms.
Step-by-step explanation:
The first step to solve this problem is to know the molecular formula of pyridine.
The molecular formula of pyridine is
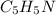
This means that in one molecule of pyridine there are 5 atoms of carbon (C), 5 atoms of hydrogen (H) and 1 atom of nitrogen (N).
Also we can state the same relation between the atoms and the moles.
1 mole of pyridine contains 5 moles of carbon, 5 moles of hydrogen and 1 mole of nitrogen.
If we want to know how many carbon atoms are in 7.05 moles of pyridine we need to know how many atoms are in one mole.
The number of atoms in one mole of any substance is the Avogadro number.
The Avogadro number is
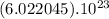
For example, in 1 mole of carbon there are
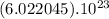 atoms of carbon.
atoms of carbon.
If we want to know the number of carbon atoms in 7.05 moles of pyridine we need to multiply :
![[(6.022045).10^(23)].(5moles).(7.05moles)=(2.123).10^(25)](https://img.qammunity.org/2018/formulas/chemistry/high-school/c1i2lepe37t0mbuvkoufzy02s0kyvzfjdc.png)
The first number is the Avogadro number
The second one is the number of carbon moles in one mole of pyridine
The third number is the number of moles of pyridine
We find that in 7.05 moles of pyridine there are
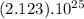 carbon atoms.
carbon atoms.