Answer: Zinc is more reactive than gold element.
Step-by-step explanation:
Single displacement reaction is defined as the reaction in which more reactive element displaces a less reactive element from its chemical reaction.
The reactivity of metal is determined by a series known as reactivity series. The metals lying above in the series are more reactive than the metals which lie below in the series.
The general chemical equation for single displacement reaction follows:
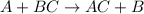
Zinc lies above in the series than gold element. Thus, when zinc metal reacts with a solution of gold ions, zinc will easily displace gold from its chemical reaction.
Hence, zinc is more reactive than gold element.