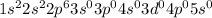Answer : The electronic configuration of Atomic number 10 is,
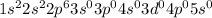
Explanation :
Electronic configuration : It is defined as the arrangement of electrons of an atom in an atomic orbitals.
In the electronic configuration, the s, p, d, f are the sub-shells which can hold 2, 6, 10, 14 electrons respectively.
Given : Atomic number = 10
Atomic number = Number of electrons
The number of electrons = 10
The electronic configuration of Atomic number 10 is,