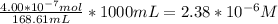The conc of NaOH is 0.104M which means that 1000mL solution contains 0.104mol of NaOH, so the amount of moles used in the titration will be
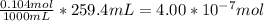
The reaction of NaOH and HCl is one to one so the moles of HCl that were titrated will also be
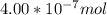
The Molarity is therefore