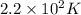Answer: Th value of A is
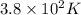 , value of B is 0.84 L, value of C is 1.1 L and the value of D is
, value of B is 0.84 L, value of C is 1.1 L and the value of D is
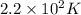
Step-by-step explanation:
To calculate the missing values of volume and temperature, we will use Charles' Law.
This law states that volume is directly proportional to the temperature of the gas at constant number of moles and pressure. Equation given by this law is:
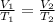 ....(1)
....(1)
where,
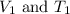 are initial volume and temperature of the gas
are initial volume and temperature of the gas
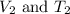 are final volume and temperature of the gas
are final volume and temperature of the gas
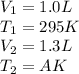
Putting values in equation 1, we get:
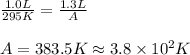
Whenever multiplication and division are involved, the answer must not contain, more number of significant figures as there are in the least precise term.
Hence, the value of A is
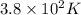
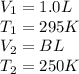
Putting values in equation 1, we get:
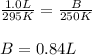
Hence, the value of B is 0.84L

Putting values in equation 1, we get:
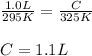
Hence, the value of C is 1.1L
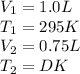
Putting values in equation 1, we get:

Hence, the value of D is