Answer: The pH of the solution is 1.34
Step-by-step explanation:
pH is defined as negative logarithm of hydrogen ion or hydronium ion concentration. It is basically defined as the power of hydrogen ions in a solution.
The equation used to calculate pH of the solution is:
![pH=-\log[H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/9so9u3mmeurzbcqkpf3jt5b89ctmc03tdp.png)
We are given:
![[H^+]=4.5* 10^(-2)M](https://img.qammunity.org/2018/formulas/chemistry/high-school/if7qgt08omh4l1apyv1weo11io1t2xh085.png)
Putting values in above equation, we get:
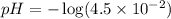
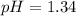
Hence, the pH of the solution is 1.34