Answer : The number of moles of
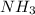 produced can be 16 moles.
produced can be 16 moles.
Solution : Given,
Moles of
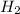 = 24.0 mol
= 24.0 mol
The balanced chemical reaction is,
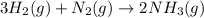
By the stoichiometry, we can say that 3 moles of hydrogen gas react with 1 mole of nitrogen gas to give 2 moles of ammonia gas as a product.
From the balanced reaction we conclude that
As, 3 moles of
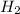 react to give 2 mole of
react to give 2 mole of
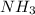
So, 24.0 moles of
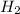 react to give
react to give
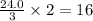 moles of
moles of
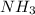
Therefore, the number of moles of
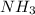 produced can be 16 moles.
produced can be 16 moles.