Answer:
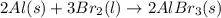
Explanation: Entropy is the measure of randomness or disorder of a system. A system has positive value of entropy if the disorder increases and a system has negative value of entropy if the disorder decreases.
Solids have highest order followed by liquids and gases have least order.
1.
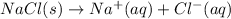
As a solid is dissociating into ions, the randomness is increasing and the entropy is also increasing.
2.
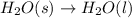
As solid is changing to liquid phase, the randomness is increasing and the entropy is also increasing.
3.
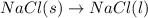
As solid is changing to liquid phase, the randomness is increasing and the entropy is also increasing.
4.
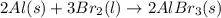
As one solid and liquid is changing into solid only, the randomness is decreasing and the entropy is also decreasing.
5.
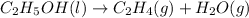
As liquid is changing to gaseous form, the randomness is increasing and the entropy is also increasing.