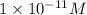Answer : The correct option is, (C)
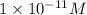
Explanation : Given,
pH = 11
pH : It is defined as the negative logarithm of hydrogen ion cocnetration.
Mathematically it is represented as :
![pH=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/y1nlg9qxar6fauop1r05a1g4xt6dhnvirc.png)
Now put the value of pH in this expression, we get the hydrogen ion concentration.
![11=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/cotfrdtw3x377mf7ullp9r5wmp1pdkxfu2.png)
![[H^+]=1.0* 10^(-11)M](https://img.qammunity.org/2018/formulas/chemistry/high-school/up2fvixlr6q2385v9s8npzokawxsys9t2m.png)
Therefore, the hydrogen ion concentration is