Answer: The boiling point of a 0.743m aqueous solution of KCl is
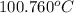 .
.
Step-by-step explanation:
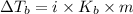
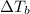 = change in boiling point
= change in boiling point
i= Vant hoff factor =is the ratio of observed colligative property to the calculated colligative property.
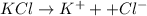
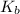 = boiling point constant for water = 0.512 °C kg/mol
= boiling point constant for water = 0.512 °C kg/mol
m= molality
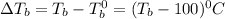
![(T_b-100)^0C=2* 0.512* 0.743}[/tex ]</p><p>[tex]T_b=100.760^C](https://img.qammunity.org/2018/formulas/chemistry/high-school/q1br0jt46xgbflcqckmoyxiz1pap0l0a7a.png)
The boiling point of the solution is
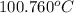 .
.