Answer: It determines the concentration of an unknown substance in neutralization reactions.
Explanation: Titration is defined as the process where a solution of known concentration is used to determine the concentration of an unknown solution.
Volumetric titrations are usually used for neutralization reactions.
When an acid and base reacts to give salt and water, the reaction is called as neutralization reaction.
The normality equation used during the titration is:
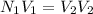
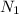 = Normality of acid solution
= Normality of acid solution
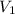 = volume of acid solution
= volume of acid solution
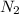 = Normality of basic solution
= Normality of basic solution
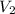 = Volume of basic solution
= Volume of basic solution