Step-by-step explanation:
Atomic mass means the sum of total number of protons and neutrons present in an element.
Molar mass is defined as the mass of one mole of a compound present in grams.
For example, molar mass of
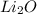 is as follows.
is as follows.
Molar mass of
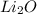 = 2 × atomic mass of Li + atomic mass of O
= 2 × atomic mass of Li + atomic mass of O
=
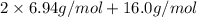
= 13.88 g/mol + 16.0 g/mol
= 29.88 g/mol
Thus, we can conclude that molar mass of
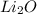 is 29.88 g/mol.
is 29.88 g/mol.